Staphylococcus aureus is a Gram-positive pathogen responsible for healthcare- and community-related infections (pneumonia, osteomyelitis, endocarditis, septic shock, etc.). S. aureus is able to develop resistance against many classes of antibiotics, and hence successfully escape most therapeutic options. Developed strategies to fight refractory staphylococcal infections include combination therapy and the use of last-resort antibiotics. However, resistance to these drugs is starting to emerge globally, depleting the options to successfully treat staphylococcal infections.
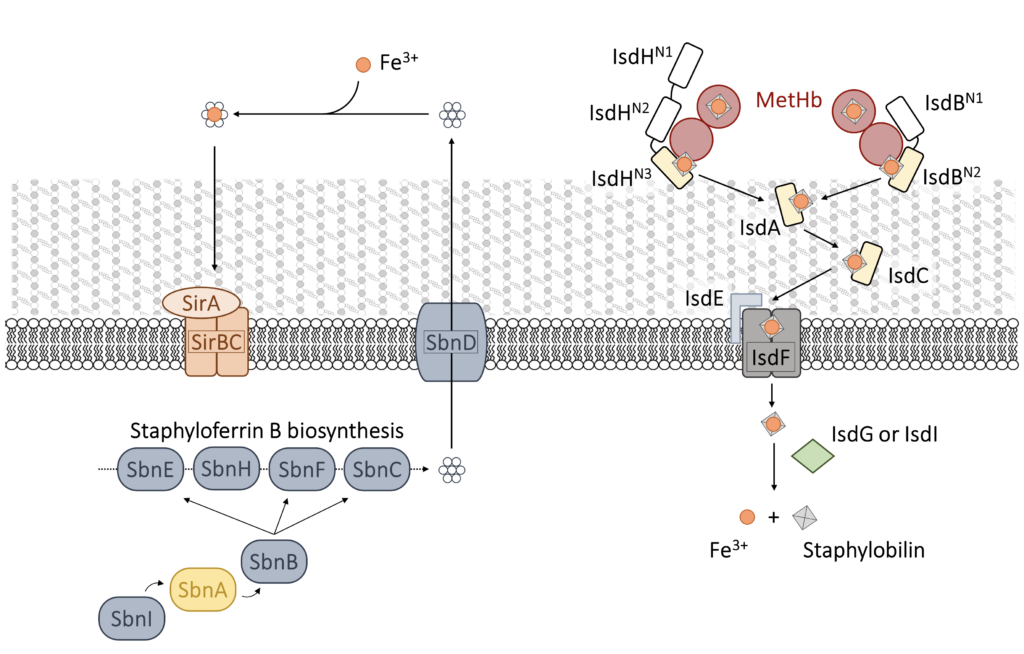
S. aureus relies on iron for host invasion and infection establishment. The bacterium possesses at least three mechanisms to get iron supply from the host: siderophores, transferrin/lactoferrin receptors and hemophores. The preferred one is heme capture from the host hemoglobin (Hb) by the Iron-regulated surface determinant (Isd) system, composed of nine proteins that bind Hb, extract heme, transfer it through the cell wall and the membrane and oxidize it to release iron (Fig. 1).
The first committed step in heme capture is carried out by two isofunctional, cell-wall anchored proteins: IsdB and IsdH. IsdH has been studied in more detail in the past, but IsdB is likely the major player in heme acquisition during infection and has been identified as a S. aureus virulence factor. These proteins exploit NEAT (NeAr iron Transporter) domains to selectively bind human Hb and to extract heme that is then transferred to other proteins of the system. Both IsdB and IsdH bind Hb by one NEAT domain through a specific interaction involving conserved Tyr residues and capture heme with another NEAT domain (Fig. 2).
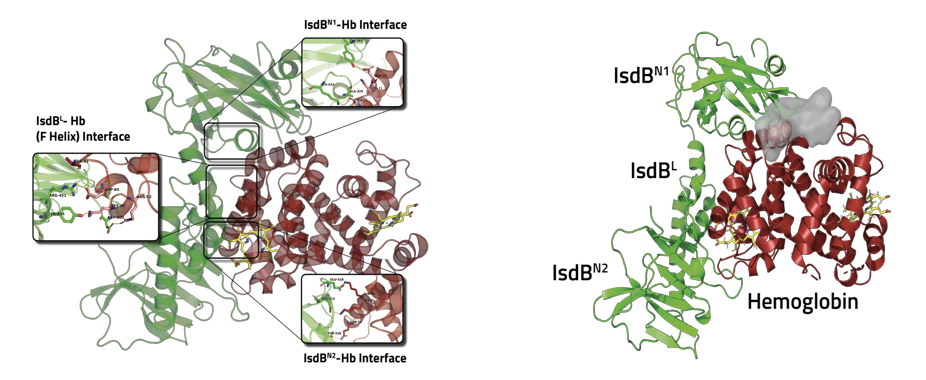
Structural studies set the ground to identify protein-protein interaction (PPI) surfaces and prompted our group to begin an in silico/in vitro study with the aim of identifying potential PPI inhibitors able to affect the first step of hemophore-Hb recognition and thus, the consequent heme extraction.
We have already identified by virtual screening molecules that target IsdB-Hb interaction and we are currently testing them for their ability to destabilize the complex by an in-house developed ELISA assay, SPR and STD-NMR.
Besides the Isd system, an additional mechanism for iron supply, the production of siderophores (staphyloferrin A, SA, and staphyloferrin B, SB), is induced during infection (Fig. 1). The first committed step of SB biosynthesis is carried out by SbnA, a PLP-dependent enzyme that catalyzes the beta-substitution of O-phosphoserine (OPS) with glutamate. Our experience in the enzymology of PLP-dependent enzymes and in their exploitation as potential targets for innovative antibacterials can be applied to the search of SbnA inhibitors.
Attacking two iron acquisition mechanisms will increase the chance to develop new antibacterials that might be also used in combination.
